AAV vs. Lentiviral Vectors: Key Differences & Applications
Includes comparison between AAV and lentiviral vector systems.
In gene and cell therapy, Adeno-Associated Viral vector (AAV) and Lentiviral Vector (LVV) are the two most widely used delivery platforms.
Both have advanced from research to the clinic (and beyond), but each brings unique strengths - and tradeoffs. Understanding when to use AAV vs. LVV is critical for matching the right tool to the right therapeutic problem.
AAV at a Glance
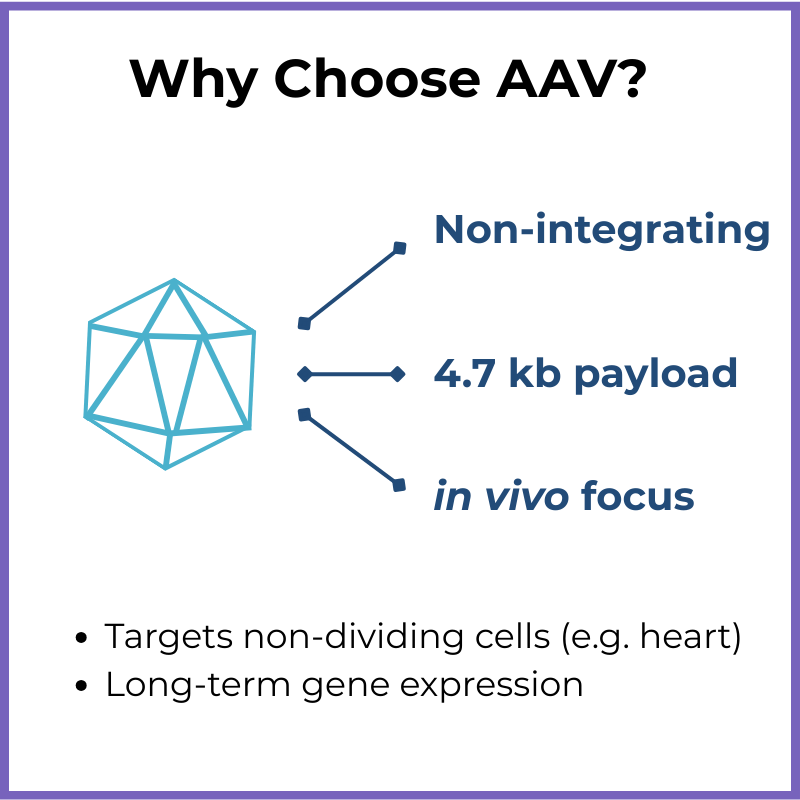
Key Features:
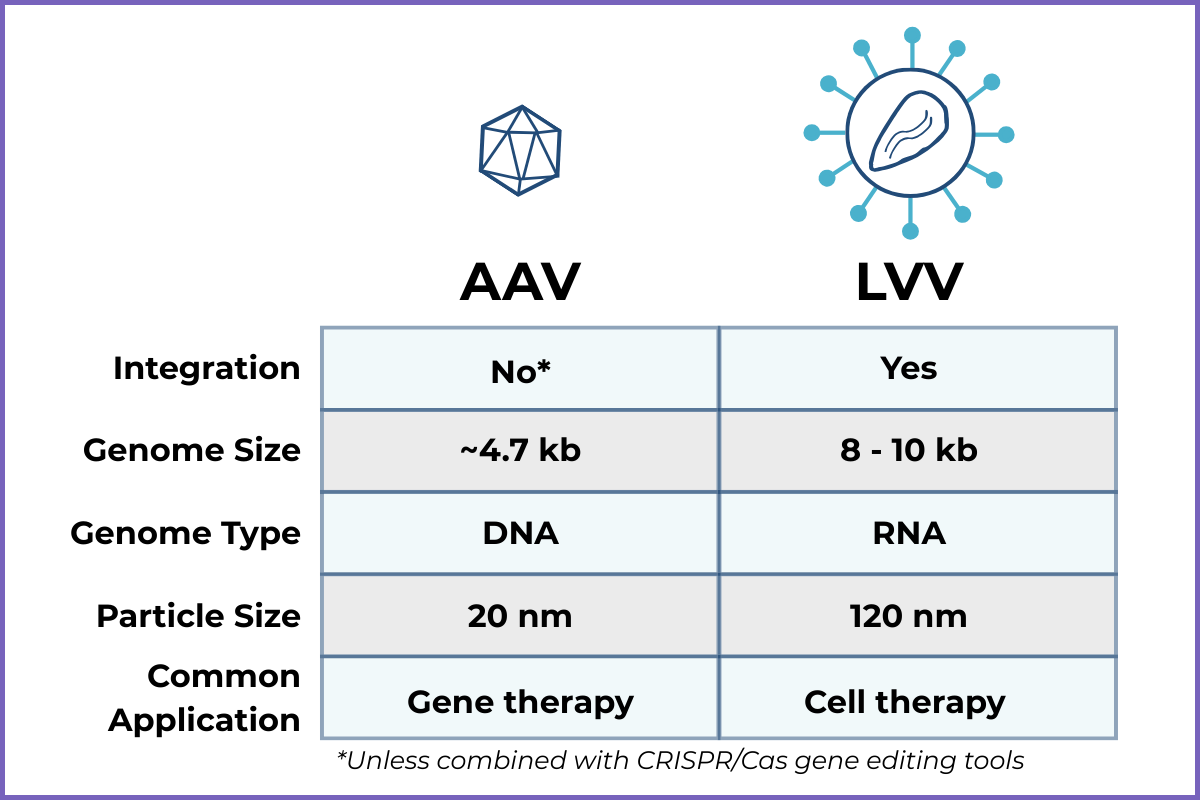
Non-integrating (remains mostly episomal, unless delivered in combination with CRISPR/Cas gene editing tools)
Can target a range of tissues depending on serotype…and the serotype can be engineered
Packaging capacity: ~4.7 kb genome
Strengths:
Strong track record with gene therapies (think: Luxturna)
Low risk of insertional mutagenesis
Limitations:
Small payload size (especially limiting when you want to deliver >1 gene)
Pre-existing immunity in many patients
May require re-dosing, which can be complicated by immune response
Lentiviral Vectors at a Glance
Key Features:
Derived from HIV-1, but replication-incompetent and engineered for safety
Genome integrates at random sites into host DNA
Packaging capacity: ~8-10 kb genome
Strengths:
Proven to be very efficient for ex vivo modification of cells (e.g. CAR-T therapy)
Larger payload capacity than AAV (potential to transfer 2+ genes)
Stable, long-term expression once integrated
Limitations:
Integration raises safety considerations (though modern designs mitigate risks)
Manufacturing can be more complex and costly
Side-by-Side Comparison Table
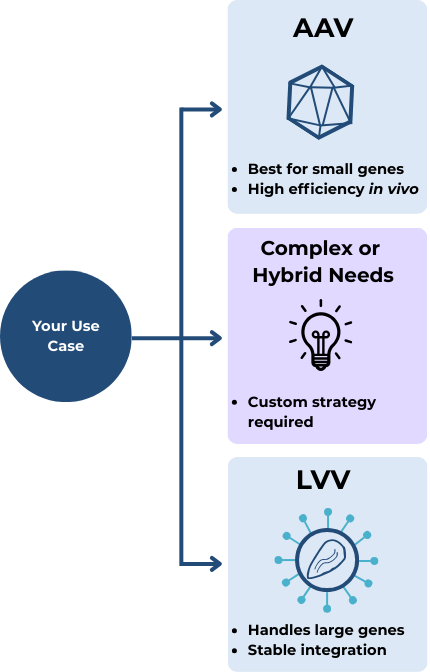
When to Use Which
Choose AAV if:
You need to deliver a small gene directly to patient tissue (in vivo)
Long-term expression without integration is acceptable…
…Or you intend to do gene editing (in combination with CRISPR/Cas)
Choose LVV if:
You’re modifying cells ex vivo and you want a system that’s plug-and-play (…or at least as much as you can get in cell & gene therapy)
You need to deliver larger or more complex genes
Stable integration is necessary, but the specific site of integration doesn’t matter (i.e. you don’t need targeted gene knock-out)
What if your project doesn’t fit neatly into one of these boxes?
Maybe you’re working on gene editing, but need to deliver a large, complex payload. Or perhaps you want the benefits of CAR-T without the logistical challenges of ex vivo manufacturing.
When your use case doesn’t align perfectly with “standard” vector applications, that’s when things get truly interesting - and when a tailored strategy makes all the difference. This is where experienced guidance can help you weigh the tradeoffs and identify the right path forward.
Key Takeaways
Both AAV and LVV are clinically validated and powerful - but serve slightly different roles
AAV currently dominates in vivo therapies, whereas LVV leads with ex vivo (though that landscape is changing)
Careful consideration of payload size, target cells, and clinical context helps to drive the choice
Getting in Touch
Have a question or want to explore working together?