Viral Vector Manufacturing 101
An overview of upstream, downstream, and analytical considerations in viral vector production
Viral vectors are the workhorses of gene and cell therapy manufacturing - but they are also living systems in miniature. Each batch begins inside cells, and every step in the manufacturing process from cell culture to final fill influences yield, potency, and safety. For professionals new to this space, understanding how upstream production, downstream purification, and analytics connect provides the foundation for designing robust, scalable processes.
Includes a one-page overview of viral vector manufacturing.
Upstream: Where the Vector is Made
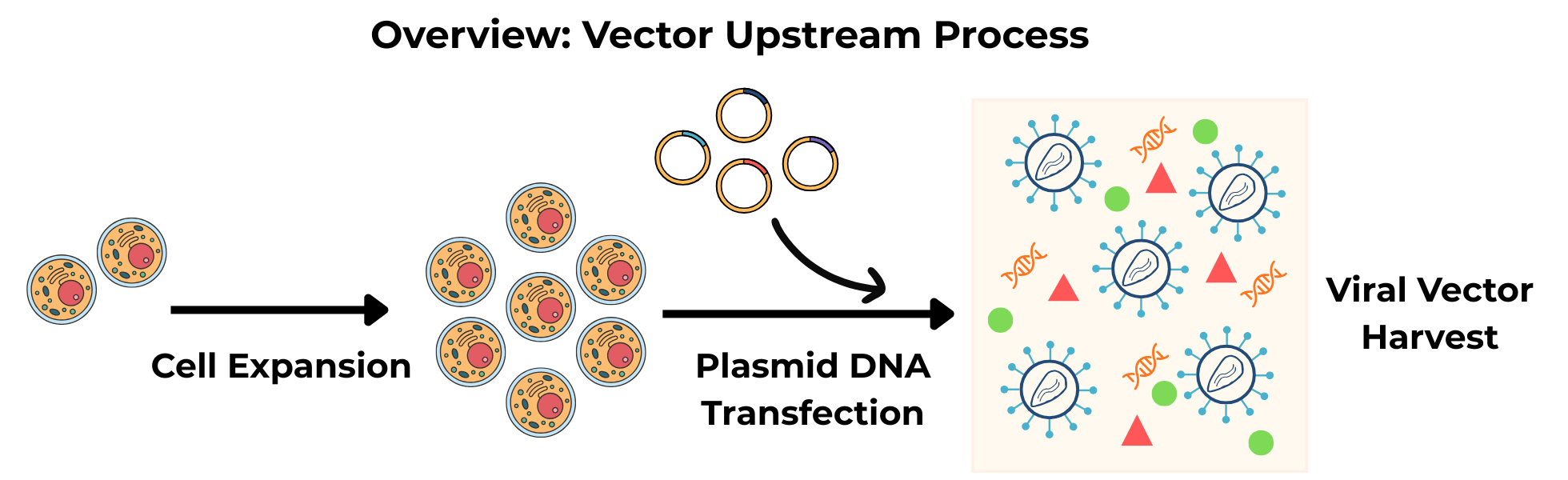
Viral vectors are produced in host cells - most commonly human embryonic kidney (HEK) cells. During upstream manufacturing, those cells are grown and then “instructed” to make vector particles. Two main production approaches dominate today:
Transient transfection, in which plasmid DNA encoding vector components is introduced into HEK cells for each batch
Stable producer cell lines, in which some or all of the necessary vector components are built into the cell genome for continuous vector production
Transient systems are flexible and fast to implement, whereas stable cell lines offer greater consistency and scalability once established.
Viral vectors are made inside host cells. Upstream manufacturing includes cell growth, transfection or induction, and harvest of vector-containing material.
Details to think about:
Adherent vs. suspension cells: adherent systems work for early R7D but limit scale; suspension systems enable bioreactors and more automated production.
Harvest timing: collecting vector too early sacrifices yield, too late can compromise particle quality.
Downstream: Purifying the Vector
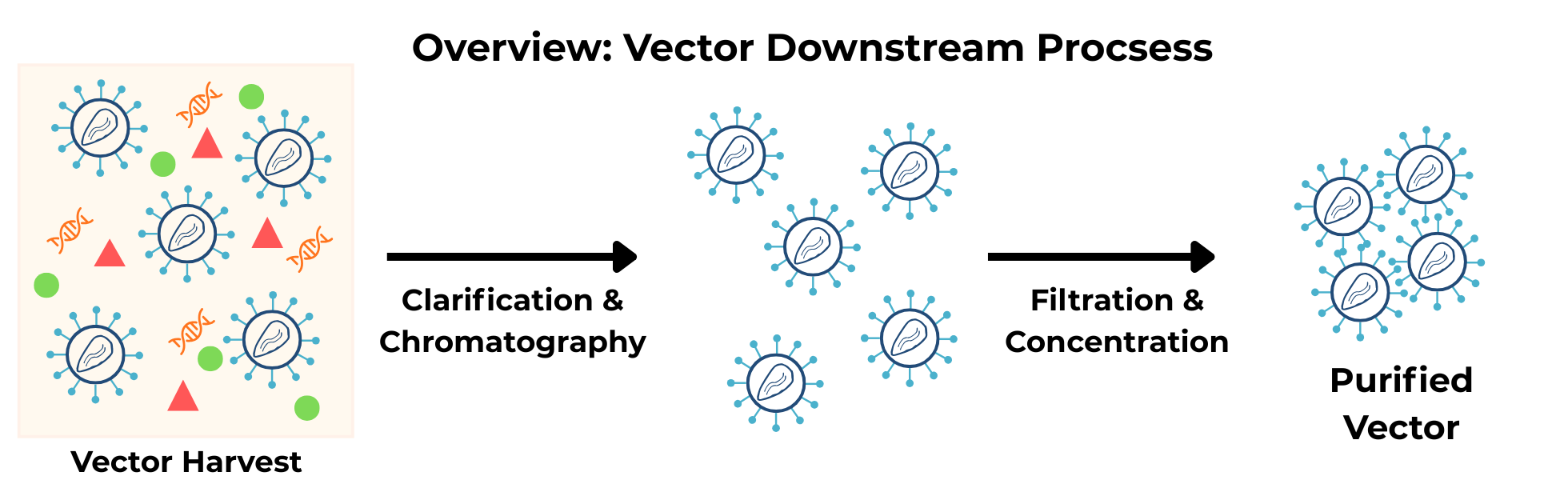
At harvest, the vector particles are present in a mixture along with cell debris, proteins, and DNA. Downstream processing isolates and concentrates functional vector. Typical steps include:
Clarification - removing cells and debris by filtration or centrifugation
Purification - using chromatography (affinity, ion-exchange, or size-based) to separate vector from impurities (e.g. host cell proteins)
Concentration and Buffer Exchange - often via tangential flow filtration (TFF) to reach the final formulation
Note: While ultracentrifugation is still often used for purification and/or concentration, this unit operation is difficult to scale and is generally replaced by chromatography for larger-scale clinical manufacturing.
Downstream processing removes impurities and concentrates functional vector. Typical steps include clarification, chromatography and filtration.
Details to think about:
Process choices depend on vector type: AAV vs. lentiviral vector (LVV) require different chromatography resins and filtration steps
Empty/full particle separation: critical for improving AAV quality
Scalability: each purification step must balance yield, purity, and cost
Analytics: Measuring What You Made
Analytical testing verifies that the manufacturing process delivers a consistent, potent, and safe product. In many ways, it is inseparable from the process itself - without the right assays, it’s impossible to tell the quantity or quality of vector that’s been produced.
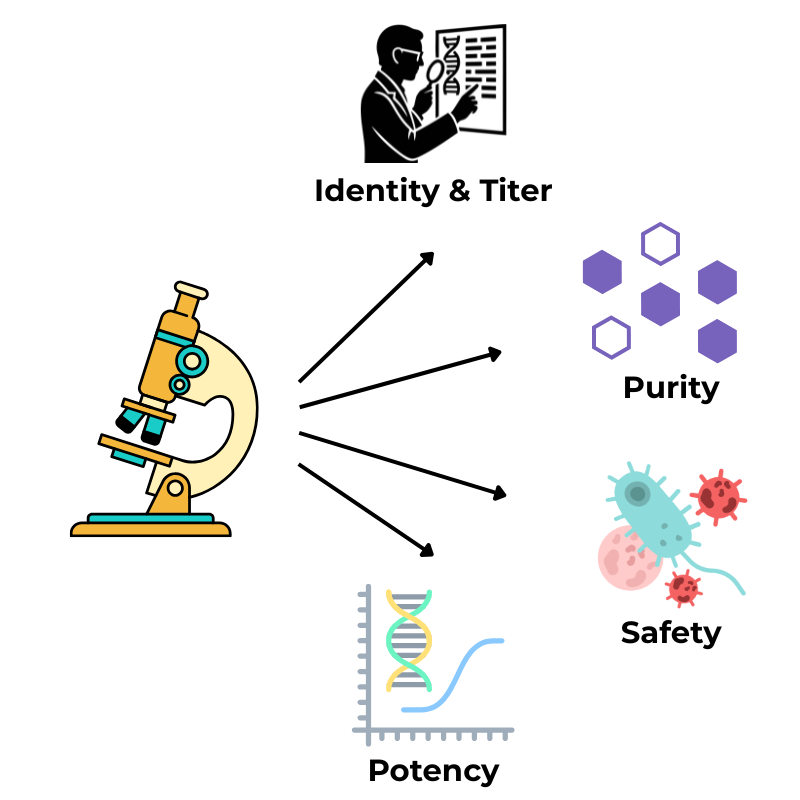
Core analytical areas include (but are not limited to):
Identity and titer: quantifying vector particles, viral genomes, and infectious particles
Purity: measuring host-cell proteins, DNA, and the ratio of full to empty capsids
Safety: testing for sterility, endotoxin, and replication-competent virus
Potency: assessing functional transduction or gene expression in relevant target cells
Details to think about
Phase-appropriate analytics: early development focuses on rapid, informative assays; later phases require higher precision and documentation. For all phases, a scientifically sound analytical approach is required
Building an analytical toolkit: select complementary methods that together describe yield, purity, and function
Analytics and process are inseparable: meaningful process optimization depends on assays you trust and understand
Why Manufacturing Strategy Matters
Small process choices - such as moving from adherent to suspension culture or changing a chromatography resin - can shift vector quality and potency. Without the right analytical tools, these changes may go undetected. The best manufacturing strategies pair process expertise with a strong analytical package that reflects an understanding of biology…or virology in this case!
Key Takeaways
Viral vector manufacturing comprises upstream production, downstream purification, and analytics
Viral vectors are made in cells; how those cells are grown determines scalability
Purification strategies must balance yield, purity, and practicality
Analytics translate process data into product understanding - the two cannot be separated
For an overview of analytical methods used to measure vector yield and potency, see How to Measure Viral Vector Titer.
Getting in Touch
Have a question or want to explore working together?